Products
TurboReader™ Point-of-Care Instrument

A compact Nephelometric diagnostic reader with barcode scanner & touch screen graphical display that performs measurements in minutes using disposable cuvettes. Easy-to-use and is compatible with serum, plasma or whole blood. Precision & accuracy comparable to routine analyzers found in central laboratories.
Specifications:
Time to Result: 3-6 min
Throughput: 15 /hr
External printer: optional
Tests: CRP, SAA, HPT, (Hb)
Size: H12 D14 W22 cm
Weight: 1 kg
Voltage: 100-240 V
Art. No. 2200-05
Equine ALB TurboReader™ Assay

An in vitro immune nephelometric assay for the quantitative determination of Albumine (ALB) in horse serum or plasma. ALB is a useful tool for assesing liver and kidney diseases. Its normal plasma concentrations in healthy horses is 26-37 g/l.
Specifications:
Species: Horse (equine)
Sample: Serum or Plasma
Sample volume: 10 μl
Range:5.0 – 75.0 g/l
Time to Result: 3 min
Kit size: 20 tests
Control: Sold separately
Art. No. 2535-01
Canine CRP TurboReader™ Assay

An in vitro immuno nephelometric assay for the quantitative determination of Canine C-Reactive (CRP) in dog serum or plasma. Canine CRP is real-time marker for systemic inflammation and infection, as well as, response to ongoing clinical treatment. Normal serum concentrations in healthy dogs is well below 35 mg/l.
Specifications:
Species: Dog (canine)
Sample: Serum or Plasma
Sample volume: 20 μl
Range: 5 – 300 mg/l
Time to Result: 3 min
Kit size: 40 tests
Control: Sold separately
Art. No. 2530-01
Equine U-ALB TurboReader™ Assay

An in vitro immune nephelometric assay for the quantitative determination of Albumine (U-ALB) in horse urine. U-ALB is a useful tool for assesing kidney function in horses. Its normal urine concentrations in healthy horses is <20-25 mg/l.
Specifications:
Species: Horse (equine)
Sample: Urine
Sample volume: 25 μl
Range:25 – 375 mg/l
Time to Result: 3 min
Kit size: 20 tests
Control: Sold separately
Art. No. 2535-02
Feline Hp TurboReader™ Assay

An in vitro immuno nephelometric assay for the quantitative determination of Haptoglobin (Hp) in cat serum or plasma. Measurement of Hp is used as a long-term monitor of inflammation or presence of subclinical disease. Low Hp indicates hemolytic anemia. Its normal concentration in healthy cats is <250 mg/dl.
Specifications:
Species: Cat (feline)
Sample: Serum or Plasma
Sample volume: 20 μl
Range:17 – 1000 mg/dl
Time to Result: 3 min
Kit size: 20 tests
Control: Sold separately
Art. No. 2532-01
EurisTest™ Turbidimetric Assay cCRP
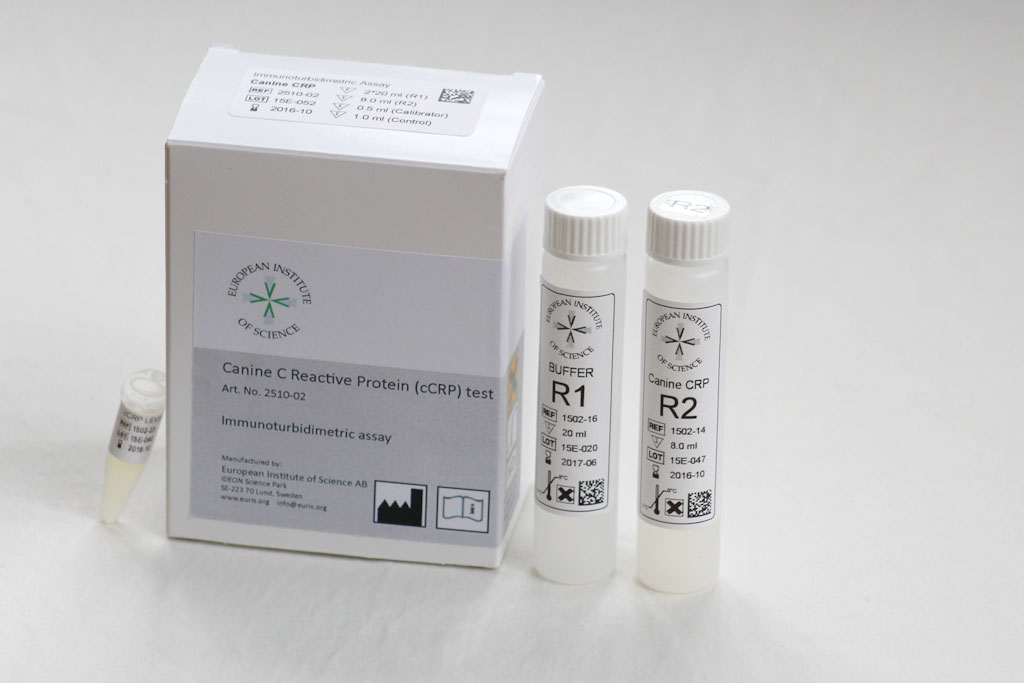
For quantitative, in vitro determination of Canine of Canine C-Reactive (CRP) in dog plasma or serum, as a tool for monitoring systemic inflammation and managing ongoing clinical treatment. Reagent kit is used with the large clinical analyzers (Abbott Architect, Olympus etc) in central labs.
Specifications:
Species: Dog (canine)
Sample: Serum or Plasma
Sample volume: 15 μl
Range: 5 – 200 mg/l
Time to Result: 10 min
Kit size: 100 tests
Control: Sold separately
Art. No. 2510-02
Feline SAA TurboReader™ Assay

An in vitro immuno nephelometric assay for the quantitative determination of Serum Amyloid A (SAA) in cat serum or plasma. SAA is a sensitive and early acute phase protein in cats peaking around 24 hours after onset of systemic inflammation or tissue injury. Its normal plasma concentrations in healthy cats is <10 mg/l.
Specifications:
Species: Cat (feline)
Sample: Serum or Plasma
Sample volume: 10 μl
Range: 10 – 225 mg/l
Time to Result: 9.5 min
Kit size: 20 tests
Control: Sold separately
Art. No. 2533-01
Pancreas Elastase 1 Quick Canine Test

A lateral flow assay for detecting canine pancreatic elastase1 in stool samples. This test allows for the fast and reliable diagnosis or exclusion of Exocrine Pancreatic Insufficiency (EPI). A positive result indicates normal exocrine pancreatic function. A negative result indicates EPI.
Specifications:
Species: Dog (canine)
Sample: Stool
Sample volume: Use extraction tool
Result: Visual
Time to Result: 10 min
Kit size: 5 tests
Art. No. SCH-25-I
Equine SAA TurboReader™ Assay

An in vitro immune nephelometric assay for the quantitative determination of Serum Amyloid A (SAA) in horse serum or plasma. SAA is an early and sensitive inflammatory marker for detecting and monitoring disease activity and response to treatment therapy. Its normal plasma concentrations in healthy horses is <20 mg/l.
Specifications:
Species: Horse (equine)
Sample: Serum or Plasma
Sample volume: 10 μl
Range:10 – 600 mg/l
Time to Result: 9.5 min
Kit size: 20 tests
Control: Sold separately
Art. No. 2534-01
Abacus Vet5 Hematology Analyzer

Vet5 offers 5-part WBC differential results for animals. Features a touch screen and an intuitive user interface for veterinary clinics and research which require a high-quality hematology analyzer. 5-part: dog, cat, horse and cattle. 3-part: mouse, rat, rabbit, ferret, pig, sheep, goat, guinea pig & primates
Specifications:
Throughput: 25 /hr
Parameters: 22 parameter CBC w/ 5-part WBC diff & 4 cellular histograms
Technology: Impedance
Sample: 25/50 µl EDTA
Reagent: Vet5 pack (200 cycles) & blood controls
Art. No. DIT-AV5
About Us
The European Institute of Science AB is a manufacturer of diagnostic assays for the clinical laboratory and point-of-care settings, as well as, point-of-care instrumentation for diagnostic applications. Our mission is to provide veterinary and medical professionals with high-quality and accurate diagnostic tests and point-of-care instrumentation required for health care needs.
With a strong focus on veterinary health, the company’s primary focus is diagnostic assays and instrumentation for canine, feline and equine animals. In order to provide a complete veterinary product line, the European Institute of Science AB is also a distributor within Scandinavia for veterinary diagnostic products produced by external manufacturers.
The European Institute of Science AB has been a unique center of excellence within innovation and entrepreneurship for more than 25 years. Together with public financing provided by the company’s shareholders, the Institute has been able to create and develop more than ten high-tech patent families and the following four public companies: Biotech-IgG/Chemel AB (founded 1996), Genovis AB (founded 1999), Implementa Hebe AB (founded 2000) and LifeAssays AB (founded 2000). The company is situated at the IDEON research park in Lund, Sweden where it has a sales office and research facilities. Additionally, the company has production facilities near the international airport Kastrup in Denmark.
Quality Policy
The management at the European Institute of Science AB is committed to maintaining a comprehensive Quality System for the development, production and sales of safe & effective, high-quality diagnostic products. We achieve our commitment to quality by complying with all applicable regulatory requirements and by striving towards continuous improvement and high customer satisfaction.
Investors
Movies
TurboReader demonstrationsfilm den 16 mars 2018: TurboReader
Investeringsdagen Stockholm den 9 december 2019: Vd Presentation
History
The European Institute of Science Inc. is a public shareholding company, which has been in existence for more than 25 years. Inventor & founder,dr. Dario Kriz, started the company in Malmoe, Sweden during 1990 with the core business centered around subcontracting and resale of laboratory equipment to chemical laboratories. During the first five years, the Institute built-up a mechanical & electronic workshop and a broad international network of subcontractors. A standard of professional craftsmanship was developed which is evident in today’s prototype constructions.
During 1994 the Institute established itself in the Science park IDEON (Lund, Sweden). This transfer of the activities from the location in Malmoe showed to be a strategically very successful step. Almost instantaneously, the Institute was carried along in a wave of intensive high-tech research resulting in several unique products. Four different off-spring companies have been created around medical and biotechnical products developed within the Institute’s research facilities: Biotech-IgG AB (Chemel AB), Genovis AB, Implementa Hebe AB, and LifeAssays AB.
The Institute went public during the fall of 1998 by conducting a public share issue in Sweden. The raised capital was used to help establish the European Institute of Science as a center of excellence in medical & biotechnology seen today. The know-how which has been built-up in the company since its foundation is currently used to develope diagnostic assays for the clinical laboratory and point-of-care settings, as well as, point-of-care instrumentation for diagnostic applications.
Management & Directors
Mikael Ehrman
Mikael Ehrman, born 1962, is the CEO and a board member since 2024.
dr. Dario Kriz
dr. Dario Kriz, born in 1965, was a board member from 1990 to 2024 and is CFO / CSO since 2024. He was the president from 1990 to 2022. He became Ph.D. in Biochemistry in 1994, visiting professor at the University of Regensburg (Germany) during 1997-1998, associate professor in 2004, and Honorary Consul for Republic of Slovenia to Kingdom of Sweden in 2015. He is the inventor of several products and methods in medical technology and biotechnology. He has 25 scientific publications and 13 approved pat686ents. He has been CEO, chairman and board member of several stock listed companies. Together with his family, he holds 33 792 shares of class A and 14 000 shares of class B in European Institute of Science AB. Personal homepage: www.linkedin.com
Magnus Nilsson
Mikael Ehrman, born 1966, is a board member since 2024.
Success Record
Biotech-IgG AB (publ)
Since 1997, European Institute of Science has been involved in developing industrial and laboratory small molecule and IgG analysis systems for the company Biotech-IgG (previously Chemel) AB, Lund (Sweden). Our success record in this particular case involved the securing of funding in the amount of approximately 10 million USD, development of 3 different patent families (instruments and procedures), acquiring EMC certification for instruments, and finally launching the products on the laboratory market. In 2009, the company acquired a Danish diagnostic kit distribution company group and thereby gained access to a professional sales organization with a net annual sales of approximately 2 million USD. During 2011-2017, European Institute of Science AB has engaged in assisting Biotech-IgG AB to expand its business into immunodiagnostics. During 2017, European Institute of Science AB sold out its share in Biotech-IgG AB. Click here to visit the Biotech-IgG homepage.
LifeAssays AB (publ)
During the period 2000 to February 2011, European Institute of Science has been involved in developing a new Point-of-Care blood analysis system for the company LifeAssays AB, Lund (Sweden). Our success record in this particular case involved the securing of funding in the amount of approximately 10 million USD, development of 7 different patent families (instrument, reagents and locked reagent data processing), acquiring EMC & ISO certification and environmental regulation permit, preparing for human IVD-CE registration (which was obtained in June 2011), and finally launching the product on the veterinary market. The product has been used to help several thousands of dog patients and reduced the unnecessary use of antibiotics for non-bacterial infections. At the end of 2010 LifeAssays had stable and growing production and sales levels, which led to the entry of the Swedish industrialist Sten K Johnsson who acquired the majority of the shares in LifeAssays. During 2011, European Institute of Science AB sold out its share in LifeAssays AB. Click here to visit the LifeAssays homepage.
Implementa Hebe AB (publ)
Since 2000, European Institute of Science has been involved in developing laboratory magnetic stirrers with cooling and heating capabilities for the company Implementa Hebe AB, Lund (Sweden). Our success record in this particular case involved the securing of funding in the amount of approximately 0,7 million USD, development of 3 different patent families (instruments and magnetic particles), acquiring EMC certification for instruments, and finally launching the products on the laboratory market. In 2010, the company acquired a patent portfolio from European Institute of Science (magnetic field generator and magnetic peptide nanoparticles) intended for therapeutic applications. During 2006, European Institute of Science AB sold out its share in Implementa Hebe AB. Click here to visit the Implementa Hebe homepage.
Genovis (publ)
During the period 1999 to 2005, European Institute of Science has been involved in developing a new gene transfer system for the company Genovis AB, Lund (Sweden). Our success record in this particular case involved the securing of funding in the amount of approximately 2 million USD, development of 3 different patent families and design of the instrument and magnetic nanoparticles. In 2006, the Swedish industrialist Bo Håkansson acquired the majority of the shares in Genovis AB. During 2007, European Institute of Science AB gradually sold out its share in Genovis AB in order to focus on new innovation challenges. Click here to visit the Genovis homepage.
The Share
European Institute of Science AB’s share has been listed on Spotlight Stock Market since February 18, 2009 under the ticker EURI B and is traded through banks and stockbrokers.
Corporate Governance - Bolagsstyrning
European Institute of Science AB (Org. No. 556404-2769) applies the Swedish Code of Corporate Governance ("the Code") as of February 1, 2010. The Code complements the external rules which affect the Corporate Governance, which is constituted mainly by the Companies Act, Accountancy legislation and current listing agreement. European Institute of Science applies fiscal financial year 1 Jan – 31 Dec, and will account for any deviations from the Code in a Corporate Governance report which will be attached to the Annual Report.These documents are available for downloading:
Documents
- Bolagsordning - Articles of Association (in Swedish)
- Informationspolicy - Informationspolicy for EURIS (in Swedish)
- Integritetspolicy (GDPR) - Integritetspolicy for EURIS (in Swedish)
Protokoll bolagsstämmor/Minutes from General Meetings
(in Swedish)
- Extra bolagsstämma 2024-09-12
- Årsstämma 2024-06-10
- Extra bolagsstämma 2024-04-10
- Extra bolagsstämma 2023-12-05
- Årsstämma 2023-05-31
- Extra bolagsstämma 2022-11-08
- Extra bolagsstämma 2022-08-25
- Årsstämma 2022-03-25
- Årsstämma 2021-05-28
- Extra bolagsstämma 2021-02-26
Auditor (Revisor)
- Mazars AB / Anders Persson, auktoriserad revisor
Economical publications
(In swedish only)
| 2024 | Q1 | Q2 | Q3 | ||
|---|---|---|---|---|---|
| 2023 | Q1 | Q2 | Q3 | Q4 | Year |
| 2022 | Q1 | Q2 | Q3 | Q4 | Year |
| 2021 | Q1 | Q2 | Q3 | Q4 | Year |
| 2020 | Q1 | Q2 | Q3 | Q4 | Year |
| 2019 | Q1 | Q2 | Q3 | Q4 | Year |
| 2018 | Q1 | Q2 | Q3 | Q4 | Year |
| 2017 | Q1 | Q2 | Q3 | Q4 | Year |
| 2016 | Q1 | Q2 | Q3 | Q4 | Year |
| 2015 | Q1 | Q2 | Q3 | Q4 | Year |
| 2014 | Q1 | Q2 | Q3 | Q4 | Year |
| 2013 | Q1 | Q2 | Q3 | Q4 | Year |
| 2012 | Q1 | Q2 | Q3 | Q4 | Year |
| 2011 | Q1 | Q2 | Q3 | Q4 | Year |
| 2010 | Q1 | Q2 | Q3 | Q4 | Year |
| 2009 | Q1 | Q2 | Q3 | Q4 | Year |
| 2008 | Q1 | Q2 | Q3 | Q4 | Year |
| 2007 | Q1 | Q2 | Q3 | Q4 | Year |
| 2006 | Q1 | Q2 | Q3 | Q4 | Year |
| 2005 | Q1 | Q2 | Q3 | Q4 | Year |
| 2004 | Q1 | Q2 | Q3 | Q4 | Year |
| 2003 | Q1 | Q2 | Q3 | Q4 | Year |
| 2002 | N/A | N/A | Q3 | Q4 | Year |
Press-releases
2024
- December 12, 2024 - Styrelsen i European Institute of Science AB (Euris) får lämna följande information till aktiemarknaden
- December 2, 2024 - Styrelseledamöterna Darijo Kriz och Rikard Krook har genom eget utträde lämnat bolagets styrelse
- November 19, 2024 - E-handeln apotek365 lanseras nu i Finland
- November 6, 2024 - Flaggningsmeddelande i European Institute of Science AB
- October 23, 2024 - Delårsrapport 2024-01-01 till 2024-09-30
- October 23, 2024 - Bolaget tidigarelägger publicering av delårsrapport 2024 Q3
- October 16, 2024 - Flaggningsmeddelande i European Institute of Science AB
- October 16, 2024 - Registrering av nyemission samt utgivande av teckningsoptioner
- October 16, 2024 - Redovisning av nettoomsättningsökning med 147 % för tredje kvartalet 2024
- September 12, 2024 - EURIS byter Vd
- September 12, 2024 - Kommuniké extra bolagsstämma 2024-09-12
- August 23, 2024 - Utökat material inför extra bolagsstämma 2024-09-12
- August 15, 2024 - Kallelse till extra bolagsstämma i European Institute of Science AB
- August 13, 2024 - Styrelsen kallar till en extra bolagsstämma med förslag till apportemission samt bemyndigande
- July 30, 2024 - Bolaget tecknar avsiktsförklaring att genom en inkråmstransaktion förvärva e-handeln apotek365
- July 30, 2024 - EURIS byter Vd
- July 10, 2024 - Halvårsrapport 2024-01-01 till 2024-06-30
- July 9, 2024 - Bolaget tidigarelägger publicering av delårsrapport 2024 Q2
- June 10, 2024 - Kommuniké årsstämma 2024
- May 26, 2024 - BTA omvandling emission
- May 16, 2024 - Förslag Dagordning/Beslut årstämma 2024
- May 14, 2024 - Flaggningsmeddelande
- May 14, 2024 - Flaggningsmeddelande
- May 14, 2024 - Utfall emission
- May 10, 2024 - Kallelse till årsstämma i European Institute of Science AB
- April 30, 2024 - Delårsrapport 2024-01-01 till 2024-03-31
- April 22, 2024 - Publicering av Emissionsmemorandum
- April 10, 2024 - Kommuniké extra bolagsstämma
- March 28, 2024 - Redovisning av nettoomsättningen för första kvartalet 2024
- March 11, 2024 - Rättelse av Kallelse till extra bolagsstämma i European Institute of Science AB
- March 9, 2024 - Kallelse till extra bolagsstämma i European Institute of Science AB
- March 6, 2024 - Styrelsens förslag till företrädesemission på 4.166 MSEK inför extra bolagsstämma
- January 31, 2024 - Bokslutskommuniké 2023-01-01 till 2023-12-31
- January 8, 2024 - Distributionsavtal för Italien tecknat med FIORAVANTI AGENCY
2023
- December 5, 2023 - Kommuniké extra bolagsstämma
- November 3, 2023 - Kallelse till extra bolagsstämma i European Institute of Science AB
- November 2, 2023 - Distributionsavtal för Spanien och Portugal tecknat med EUROVET VETERINARIA S.L.
- October 31, 2023 - Delårsrapport 2023-01-01 till 2023-09-30
- August 31, 2023 - Halvårsrapport 2023-01-01 till 2023-06-30
- August 24, 2023 - Flaggningsmeddelande i European Institute of Science AB
- August 1, 2023 - Senarelagd Q2
- June 6, 2023 - Beslut avstämningsdag rättelse
- June 6, 2023 - Beslut avstämningsdag
- May 31, 2023 - Kommuniké årsstämma 2023
- May 10, 2023 - Årsredovisning för verksamhetsåret 2022
- May 2, 2023 - Kallelse till årsstämma i European Institute of Science AB
- May 2, 2023 - Styrelsens förslag till beslut om sammanläggning av aktier
- April 28, 2023 - Delårsrapport 2023-01-01 till 2023-03-31
- January 31, 2023 - Rättelse av bokslutskommuniké 2022-01-01 till 2022-12-31 EURIS 2022 Q4
- January 31, 2023 - Bokslutskommuniké 2022-01-01 till 2022-12-31
- January 23, 2023 - OEM avtal med Staperia srls
- January 20, 2023 - OEM avtal med CSR Test Easy ltd
2022
- December 22, 2022 - BTU omvandling emission
- December 8, 2022 - Övertecknad nyemission
- December 1, 2022 - Tillägg till memorandum
- November 21, 2022 - Flaggningsmeddelande i European Institute of Science AB
- November 21, 2022 - Flaggningsmeddelande i European Institute of Science AB
- November 14, 2022 - Publicering av Emissionsmemorandum
- November 8, 2022 - Kommuniké från extra bolagsstämma 2022-11-08
- October 24, 2022 - Flaggningsmeddelande i European Institute of Science AB
- October 24, 2022 - Delårsrapport 2022-01-01 till 2022-09-30
- October 23, 2022 - Bolaget tidigarelägger publicering av delårsrapport 2022 Q3
- October 10, 2022 - Rättelse av kallelse till extra bolagsstämma i European Institute of Science AB
- October 10, 2022 - Rättelse av pressmeddelande: Styrelsens förslag till företrädesemission på 4.166 MSEK
- October 7, 2022 - Rättelse av kallelse till extra bolagsstämma i European Institute of Science AB
- October 6, 2022 - Rättelse av pressmeddelande: Styrelsens förslag till företrädesemission på 4.166 MSEK
- October 6, 2022 - Kallelse till extra bolagsstämma i European Institute of Science AB
- October 6, 2022 - Styrelsens förslag till företrädesemission på 4.166 MSEK
- August 25, 2022 - Kommuniké från extra bolagsstämma
- August 11, 2022 - EURIS byter Vd
- July 15, 2022 - Kallelse till extra bolagsstämma i European Institute of Science AB
- July 13, 2022 - Halvårsrapport 2022-01-01 till 2022-06-30
- July 12, 2022 - Bolaget tidigarelägger publicering av delårsrapport 2022 Q2
- June 14, 2022 - Distributionsavtal för Spanien tecknat med CliniSciences group
- May 31, 2022 - Delårsrapport 2022-01-01 till 2022-03-31
- April 28, 2022 - Bolaget senarelägger publicering av delårsrapport 2022 Q1
- March 25, 2022 - Kommuniké årsstämma 2022
- March 4, 2022 - Årsredovisning för verksamhetsåret 2021
- February 17, 2022 - Kallelse till årsstämma i European Institute of Science AB
- January 31, 2022 - Bokslutskommuniké 2021-01-01 till 2021-12-31
2021
- November 30, 2021 - Delårsrapport 2021-01-01 till 2021-09-30
- October 28, 2021 - Bolaget senarelägger publicering av delårsrapport 2021 Q3
- July 30, 2021 - Halvårsrapport 2021-01-01 till 2021-06-30
- July 29, 2021 - Bolaget tidigarelägger publicering av delårsrapport 2021 Q2
- May 28, 2021 - Kommuniké årsstämma 2021
- May 12, 2021 - Delårsrapport 2021-01-01 till 2021-03-31
- May 12, 2021 - Bolaget tidigarelägger publicering av delårsrapport 2021 Q1
- May 11, 2021 - Rättelse årsredovisning för verksamhetsåret 2020
- May 10, 2021 - Årsredovisning för verksamhetsåret 2020
- April 29, 2021 - Bolaget senarelägger publicering av delårsrapport 2021 Q1
- April 26, 2021 - Kallelse till årsstämma i European Institute of Science AB
- April 22, 2021 - BTA omvandling emission
- March 30, 2021 - Övertecknad nyemission
- March 8, 2021 - Publicering av Emissionsmemorandum
- February 26, 2021 - Kommuniké från extra bolagsstämma 2021-02-26
- February 5, 2021 - Dagordning extra bolagsstämma i European Institute of Science AB
- January 29, 2021 - Komplettering av bokslutskommuniké 2020-01-01 till 2020-12-31
- January 29, 2021 - Bokslutskommuniké 2020-01-01 till 2020-12-31
- January 26, 2021 - Rättelse av kallelse till extra bolagsstämma i European Institute of Science AB
- January 26, 2021 - Rättelse av pressmeddelande: Styrelsens förslag till företrädesemission på 4.34 MSEK samt nyval av styrelseledamot
- January 26, 2021 - Kallelse till extra bolagsstämma i European Institute of Science AB
- January 26, 2021 - Styrelsen kallar till en extra bolagsstämma med förslag till företrädesemission på 4.34 MSEK samt nyval av styrelseledamot
2020
- December 13, 2020 - Ledamot lämnar bolaget styrelse
- November 30, 2020 - Delårsrapport 2020-01-01 till 2020-09-30
- November 24, 2020 - BTA omvandling emission
- November 18, 2020 - Bolaget senarelägger publicering av delårsrapport 2020 Q3
- November 2, 2020 - Utfall nyemission 1.78 MSEK
- October 29, 2020 - Bolaget senarelägger publicering av delårsrapport 2020 Q3
- October 14, 2020 - Publicering av Emissionsmemorandum
- October 2, 2020 - Kommuniké från extra bolagsstämma 2020-10-02
- September 28, 2020 - Bolaget har erhållit sin första order från sin svenska distributör LAB Diagnostics Nordic AB
- September 25, 2020 - Miljönämndens beslut möjliggör kostnadsbesparingar genom flytt av bolagets produktion från Danmark till Sverige
- September 8, 2020 - Distributionsavtal för Ungern tecknat med Bio-Kasztel Kft
- September 2, 2020 - Kallelse till extra bolagsstämma i European Institute of Science AB
- September 1, 2020 - Styrelsen kallar till en extra bolagsstämma med förslag till företrädesemission på 6.9 MSEK
- August 18, 2020 - Delårsrapport 2020-01-01 till 2020-06-30
- August 17, 2020 - EURIS tidigarelägger publicering av halvårsrapport (Q1-2) 2020
- May 12, 2020 - Flaggningsmeddelande
- May 12, 2020 - Flaggningsmeddelande
- May 12, 2020 - Flaggningsmeddelande
- May 12, 2020 - BTA omvandling emission
- May 8, 2020 - Delårsrapport 2020-01-01 till 2020-03-31
- April 28, 2020 - Bolaget senarelägger publicering av delårsrapport 2020 Q1
- April 20, 2020 - Utfall nyemission 1.468 MSEK
- April 14, 2020 - Ökad månatlig försäljning av bolagets blodtest under perioden 1 jan - 14 apr samt lägesuppdatering med anledning av COVID-19
- March 30, 2020 - Bolaget har erhållit sin första order från Korea
- Mars 26, 2020 - Ökad försäljning under 2020 Q1 samt lägesuppdatering med anledning av COVID-19
- Mars 20, 2020 - Publicering av Emissionsmemorandum
- Mars 20, 2020 - Kommuniké årsstämma 2020
- March 2, 2020 - Distributionsavtal för Sloveninen tecknat med Felivet d.o.o.
- February 28, 2020 - Årsredovisning för verksamhetsåret 2019
- February 14, 2020 - Kallelse till årsstämma i European Institute of Science AB
- February 14, 2020 - Styrelsen har beslutat att på årsstämman föreslå en företrädesemission på 5.42 MSEK
- February 5, 2020 - Bolaget har erhållit sin första order från nyutnämnd distributör i Italien
- January 31, 2020 - Bokslutskommuniké 2019-01-01 till 2019-12-31
- January 30, 2020 - Distributionsavtal för Frankrike och Portugal tecknat med CliniSciences group
- January 23, 2020 - Distributionsavtal för Italien tecknat med Laboratory Service s.r.l
- January 22, 2020 - Distributionsavtal för Sverige tecknat med LAB Diagnostics Nordic AB gällande försäljning av reagens till Roche cobas c 111 och c 311
- January 10, 2020 - Flaggningsmeddelande
- January 9, 2020 - BTA omvandling emission
2019
- December 20, 2019 - Flaggningsmeddelande
- December 16, 2019 - Utfall emission
- November 28, 2019 - PSL Alliance
- November 15, 2019 - Publicering av Emissionsmemorandum
- November 15, 2019 - Kommuniké från extra bolagsstämma 2019
- October 31, 2019 - Delårsrapport 2019-01-01 till 2019-09-30
- October 17, 2019 - BTA omvandling emission
- October 11, 2019 - Kallelse till extra bolagsstämma den 15:e november 2019
- October 11, 2019 - Styrelsen kallar till extra bolagstämma och föreslår en företrädesemission på 4.27 MSEK
- October 10, 2019 - Flaggningsmeddelande
- October 10, 2019 - Flaggningsmeddelande
- October 1, 2019 - Utfall emission
- September 16, 2019 - Flaggningsmeddelande
- September 6, 2019 - Blåstjärnan, ett av Sveriges ledande djursjukhus, väljer TurboReader
- September 3, 2019 - Publicering av Emissionsmemorandum
- August 22, 2019 - Korrigering beslut om nyemission
- August 22, 2019 - Beslut om nyemission
- August 21, 2019 - Halvårsrapport 2019-01-01 till 2019-06-30
- August 19, 2019 - Tidigarelagd publicering av delårsrapport jan-jun 2019
- May 17, 2019 - Kommuniké årsstämma 2019
- April 30, 2019 - Delårsrapport 2019-01-01 till 2019-03-31
- April 29, 2019 - BTA omvandling emission
- April 26, 2019 - Årsredovisning för verksamhetsåret 2018
- April 8, 2019 - Kallelse till årsstämma den 17:e maj 2019
- April 2, 2019 - Utfall emission
- March 7, 2019 - Publicering av Emissionsmemorandum
- March 1, 2019 - Beslut om nyemission
- February 15, 2019 - Lansering av två nya test för lever- och njurfunktions störningar hos häst
- February 15, 2019 - Bolagets instrument Turboreader visar positiv utvärdering på hästar
- February 14, 2019 - Valberedning och Årsstämma 2019
- February 12, 2019 - Positiv evaluering av TurboReader (katt-SAA)
- February 1, 2019 - Bokslutskommuniké 2018-01-01 till 2018-12-31
2018
- November 20, 2018 - BTA omvandling emission
- November 06, 2018 - Delårsrapport 2018-01-01 till 2018-09-30
- November 5, 2018 - Häst (SAA) test färdigt och levererat till första kund i Sverige
- October 30, 2018 - Senareläggning Q3
- October 25, 2018 - Flaggningsmeddelande
- October 23, 2018 - Utfall emission
- October 16, 2018 - Katt (SAA) test färdigt och levererat till första kund i Sverige
- September 20, 2018 - Publicering av Emissionsmemorandum
- September 11, 2018 - Beslut om nyemission
- August 31, 2018 - Kommuniké från extra bolagsstämma 2018
- August 7, 2018 - Sista dag för handel med BTA
- August 1, 2018 - Kallelse till extra bolagsstämma den 31:e augusti 2018
- July 31, 2018 - Halvårsrapport 2018-01-01 till 2018-06-30
- July 31, 2018 - EURIS tidigarelägger publicering av halvårsrapport (Q1-2) 2018
- July 16, 2018 - Bolaget erhåller order till sammanlagt värde av 75 tSEK från ny kund på Lunds Universitet
- July 6, 2018 - Flaggningsmeddelande
- July 4, 2018 - Flaggningsmeddelande
- July 3, 2018 - BIOMEDICA och European Institute of Science undertecknar Letter of Intent gällande distribution i Österrike, Tjeckien, Slovakien och Slovenien
- July 3, 2018 - Utfall emission
- Juni 25, 2018 - Ny instrument och kit beställning från distributör i Litauen
- Juni 18, 2018 - Bolaget erhåller sin första order till sammanlagt värde av 75 tSEK från ny kund på Lunds Universitet
- Juni 8, 2018 - De två första svenska djursjukhusen har börjat använda TurboReader som rutinmetod
- Juni 5, 2018 - Distributör i Litauen beställer fler instrument och kit
- Maj 24, 2018 - Publicering av Emissionsmemorandum
- Maj 15, 2018 - Beslut om nyemission
- April 26, 2018 - Flaggningsmeddelande
- April 9, 2018 - Kommuniké årsstämma 2018
- April 5, 2018 - Delårsrapport 2018-01-01 till 2018-03-31
- Mars 23, 2018 - Valberedningens förslag
- Mars 16, 2018 - Kommentar till revisionsberättelse
- Mars 16, 2018 - Årsredovisning för verksamhetsåret 2017
- Mars 5, 2018 - Kallelse till årsstämma den 6:e april 2018
- January 31, 2018 - Bokslutskommuniké 2017-01-01 till 2017-12-31
- January 23, 2018 - Distributionsavtal tecknat med Diatron MI Zrt
- January 11, 2018 - Distributionsavtal tecknat med Biopanda Reagents Ltd
- January 9, 2018 - Distributionsavtal tecknat med BHH Mikromed Sp
2017
- December 22, 2017 - Korrigering sista dag för handel med BTA
- December 21, 2017 - Sista dag för handel med BTA
- December 13, 2017 - Distributionsavtal tecknat med Sacace Biotechnologies SRI
- November 30, 2017 - Distributionsavtal tecknat med RI.MOS SRI
- November 30, 2017 - Delårsrapport 2017-01-01 till 2017-09-30
- November 10, 2017 - Valberedning och Årsstämma 2018
- November 6, 2017 - Utfall nyemission
- October 26, 2017 - Ny produktbroschyr framtagen inför EURIS besök på MEDICA mässan 13-16 november
- October 19, 2017 - EURIS senarelägger publicering av delårsrapport (Q1-3) 2017
- October 6, 2017 - Publicering av Emissionsmemorandum
- October 6, 2017 - Kommuniké från extra bolagsstämma 2017
- September 15, 2017 - Dagordning/Förslag inför extra bolagsstämma 2017-10-06
- September 7, 2017 - European Institute of Science AB utser Mangold Fondkommission AB till emissionsinstitut i samband med föreslagen företrädesemission
- September 6, 2017 - Korrigering kallelse till extra bolagsstämma
- September 5, 2017 - Kallelse till extra bolagsstämma
- August 1, 2017 - Halvårsrapport 2017-01-01 till 2017-06-30
- July 31, 2017 - EURIS tidigarelägger publicering av halvårsrapport (Q1-2) 2017
- July 3, 2017 - Försäljning Biotech-IgG aktier
- May 31, 2017 - Bolaget tecknar distributionsavtal inom veterinärdiagnostik med distributör i Danmark
- May 30, 2017 - Kommuniké årsstämma 2017
- May 24, 2017 - Delårsrapport 2017-01-01 till 2017-03-31
- April 13, 2017 - EURIS senarelägger publicering av kvartalsrapport Q1 2017
- January 31, 2017 - Bokslutskommuniké 2016-01-01 till 2016-12-31
2016
- November 30, 2016 - Valberedning och Årsstämma 2017
- November 30, 2016 - Delårsrapport 2016-01-01 till 2016-09-30
- October 25, 2016 - EURIS senarelägger publicering av delårsrapport (Q1-3) 2016
- August 31, 2016 - Halvårsrapport 2016-01-01 till 2016-06-30
- August 17, 2016 - Sista dag för handel med BTA
- Juli 5, 2016 - Utfall nyemission
- Juni 27, 2016 - European Institute of Science får exklusiv distributionsrätt i Sverige, Danmark och Norge genom avtal med ScheBo Biotech AG
- Juni 21, 2016 - Bolaget har erhållit sin andra order för sitt nya patientnära veterinärdiagnostiska system av nyutnämnd distributör i Litauen
- Juni 14, 2016 - Bolaget har erhållit sin första order för sitt nya patientnära veterinärdiagnostiska system
- Juni 7, 2016 - Publicering av Emissionsmemorandum
- Juni 3, 2016 - Kommuniké från extra bolagsstämman 2016-06-03
- Juni 1, 2016 - Bolagets tecknar sitt första distributionsavtal inom veterinärdiagnostik med distributör i Taiwan
- Maj 13, 2016 - Förslag till dagordning och beslut på extra bolagsstämma 2016-06-03
- Maj 3, 2016 - Kallelse till extra bolagsstämma den 3:e juni 2016
- April 29, 2016 - Delårsrapport 2016-01-01 till 2016-03-31
- Mars 22, 2016 - European Institute of Science öppnar Webbshop för global direktförsäljning av bolagets veterinära produkter
- Mars 11, 2016 - Dagordning/förslag inför årsstämma 2016
- Mars 4, 2016 - Bolagets instrument för veterinärdiagnostik erhåller viktig EMC CE märkning enligt EN 61326-1: 2012 och EN 61326-2-3: 2012
- February 24, 2016 - Kallelse till årsstämma den 1:e april 2016
- February 19, 2016 - Bolaget öppnar ett nytt laboratorium på IDEON
- February 1, 2016 - Distributionsavtal tecknat med Primer Design Ltd
- January 29, 2016 - Bokslutskommuniké 2015-01-01 till 2015-12-31
2015
- November 30, 2015 - Valberedning och Årsstämma 2016
- October 30, 2015 - Delårsrapport 2015-01-01 till 2015-09-30
- August 20, 2015 - Halvårsrapport 2015-01-01 till 2015-06-30
- August 20, 2015 - EURIS tidigarelägger publicering av halvårsrapport Q1-2 för 2015
- May 15, 2015 - Kommuniké årsstämma 2015
- April 29, 2015 - Delårsrapport 2015-01-01 till 2015-03-31
- April 23, 2015 - EURIS sluter ett joint venture avtal med DiaSystem Scandinavia AB
- April 23, 2015 - Dagordning/förslag inför årsstämma 2015
- April 17, 2015 - Kallelse till årsstämma den 15:e maj 2015
- April 16, 2015 - Flaggningsmeddelande i European Institute of Science AB
- January 30, 2015 - Bokslutskommuniké 2014-01-01 till 2014-12-31
2014
- December 17, 2014 - Flaggningsmeddelande i European Institute of Science AB
- December 11, 2014 - Valberedning inför årsstämma 2015
- October 20, 2014 - Delårsrapport 2014-01-01 till 2014-09-30
- October 15, 2014 - Flaggningsmeddelande i European Institute of Science AB
- September 9, 2014 - EURIS sluter ett joint venture avtal med ett bolag i USA gällande gemensam utveckling samt kommersialisering av snabbtest av lateral flow typ för veterinärmarknaden
- August 26, 2014 - Halvårsrapport 2014-01-01 till 2014-06-30
- April 30, 2014 - Delårsrapport 2014-01-01 till 2014-03-31
- Mars 18, 2014 - Flaggningsmeddelande
- February 28, 2014 - Kommuniké årsstämma 2014
- February 7, 2014 - Dagordning/förslag inför årsstämma 2014
- January 31, 2014 - Bokslutskommuniké 2013-01-01 till 2013-12-31
- January 28, 2014 - Kallelse till årsstämma den 28:e februari 2014
2013
- November 25, 2013 - Kommuniké extra bolagsstämma 2013
- October 31, 2013 - Delårsrapport 2013-01-01 till 2013-09-30
- October 28, 2013 - Kallelse till extra bolagsstämma den 25:e november 2013
- August 30, 2013 - Halvårsrapport 2013-01-01 till 2013-06-30
- May 10, 2013 - Kommuniké årsstämma 2013
- April 30, 2013 - Delårsrapport 2013-01-01 till 2013-03-31
- April 19, 2013 - Dagordning/förslag inför årsstämma 2013
- April 9, 2013 - Kallelse till årsstämma den 10:e maj 2013
- Mars 1, 2013 - Antalet utestående aktier per 2013-02-28
- February 28, 2013 - Bokslutskommuniké 2012-01-01 till 2012-12-31
- February 12, 2013 - Handel BTA avslutas
- January 30, 2013 - Senarelagd Q4
2012
- December 28, 2012 - Utfall nyemission
- November 30, 2012 - Offentliggörande av emissionsmemorandum
- November 14, 2012 - Reviderad version - beslut om nyemission med företräde för aktieägarna
- November 14, 2012 - Beslut om nyemission med företräde för aktieägarna
- October 31, 2012 - Flaggningsmeddelande i Biotech-IgG AB
- October 31, 2012 - Delårsrapport 2012-01-01 till 2012-09-30
- August 31, 2012 - Delårsrapport 2012-01-01 till 2012-06-30
- August 20, 2012 - EURIS avser öka sitt innehav i Biotech Biotech-IgG AB:s kommande företrädesemission
- May 11, 2012 - Kommuniké årsstämma 2012
- April 30, 2012 - Delårsrapport 2012-01-01 till 2012-03-31
- April 20, 2012 - Årsredovisning 2012
- April 20, 2012 - Dagordning/förslag inför årsstämma 2012
- April 13, 2012 - Kallelse till årsstämma i European Institute of Science AB
- April 12, 2012 - Styrelsen har beslutat att senarelägga framläggande av förslag till utdelning av aktier i dotterbolaget
- Mars 27, 2012 - Euris ökar sitt innehav i Biotech-IgG AB med 1 643 800 nyteckande aktier
- Mars 26, 2012 - Styrelsen förslår årsstämma 2012 att besluta om utdelning av aktier i dotterbolaget, nedskrivning av aktiekapital samt nyemissionsbemyndigande
- February 29, 2012 - Bokslutskommuniké 2011-01-01 till 2011-12-31
- January 24, 2012 - Nytt datum för bokslutskommuniké Q4 2011
2011
- December 12, 2011 - Kommuniké från extra bolagsstämma 2011-12-12
- December 2, 2011 - Styrelsens förslag till beslut gällande nyval av två styrelseledamöter vid extra bolagsstämma
- November 30, 2011 - EURIS offentliggör härmed ett debattinlägg från bolagets vd Dario Kriz
- November 28, 2011 - Försäljning LifeAssays aktier
- November 14, 2011 - Kallelse till extra bolagsstämma i European Institute of Science AB
- November 7, 2011 - Offentliggörande av investeringsmemorandum inför
dotterbolaget ScienceHouse Lund AB:s emission - October 31, 2011 - Delårsrapport 2011-01-01 till 2011-09-30
- October 31, 2011 - European Institute of Science ingår franchiseavtal med sitt helägda dotterbolag
- October 26, 2011 - European Institute of Science säljer sin fastighet samt konsulttjänster till sitt helägda dotterbolag
- October 10, 2011 - ScienceHouse i Lund placeras i helägt dotterbolag
- September 21, 2011 - Nyteckning i LifeAssays AB
- August 11, 2011 - Flaggningsmeddelande i LifeAssays AB
- July 12, 2011 - Halvårsrapport 2011-01-01 till 2011-06-30
- July 8, 2011 - Margareta Pené lämnar bolaget styrelse
- June 21, 2011 - Implementa påbörjar försäljning
- April 29, 2011 - Kvartalsrapport 2011-01-01 till 2011-03-31
- April 20, 2011 - Dagordning/förslag inför årsstämma 2011
- April 8, 2011 - Euris tecknar i Chemels emission och blir bolagets största ägare
- Februari 2, 2011 - Euris och Chemel tecknar överlåtelseavtal för proteinreningsteknik
- Januari 31, 2011 - Bokslutskommuniké 2010-01-01 till 2010-12-31
2010
- December 3, 2010 - Sista dag för handel med BTA den 7 december 2010
- November 1, 2010 - Delårsrapport 2010-01-01 till 2010-09-30
- October 13, 2010 - Nyemission tillför bolaget 483 tkr
- October 13, 2010 - Flaggningsmeddelande i LifeAssays AB
- September 8, 2010 - Försäljning av patent- och produktportfölj till Implementa Hebe AB
- September 7, 2010 - Beslut om nyemission med företräde för aktieägarna
- September 6, 2010 - Sista dag för handel med BTA den 8 september 2010
- Augusti 31, 2010 - Halvårsrapport 2010-01-01 till 2010-06-30
- July 22, 2010 - Nyemissions tillför bolaget 353 tkr
- Juni 24, 2010 - Offentliggörande av emissionsmemorandum
- Juni 18, 2010 - Korrigerad teckningstid samt tid för handel med teckningsrätter
- Juni 15, 2010 - Beslut om nyemission med företräde för aktieägarna
- Juni 3, 2010 - Återkallande av beslut om ändring av §7 i bolagsordningen
- Juni 3, 2010 - Kommuniké årsstämma 2010
- May 7, 2010 - Kvartalsrapport 2010-01-01 till 2010-03-31
- April 29, 2010 - Nytt datum för kvartalsrapport Q1 2010
- April 27, 2010 - Dagordning/förslag inför årsstämma 2010
- April 12, 2010 - Kallelse till årsstämma 2010
- Mars 26, 2010 - Förslag till emissionsbemyndigande för att säkerställa drift och pågående investeringar
- Februari 25, 2010 - Bokslutskommuniké 2009-01-01 till 2009-12-31
- Februari 15, 2010 - Nytt datum för bokslutskommuniké Q4 2009
- Februari 3, 2010 - Nytt datum för bokslutskommuniké Q4 2009
2009
- Oktober 29, 2009 - Delårsrapport 2009-01-01 till 2009-09-30
- October 1, 2009 - Chinese university buys Magnetic Field Generator
- Augusti 28, 2009 - Halvårsrapport 2009-01-01 till 2009-06-30
- Maj 19, 2009 - Patent beviljat i Japan för växlande gradientfältsinstrument.
- Maj 18, 2009 - Kommuniké årsstämma 2009
- April 28, 2009 - Kvartalsrapport 2009-01-01 till 2009-03-31
- April 16, 2009 - Kallelse till årsstämma 2009
- April 08, 2009 - Finansiering säkrad för deltagande i LifeAssays företrädesemission
- Mars 17, 2009 - Första distributionsavtal för USA tecknat med Ocean Nano Tech LLC
- Februari 11, 2009 - Bokslutskommuniké 2008-01-01 till 2008-12-31
- Februari 2, 2009 - EURIS byter handelsplats från NGM Equity till Aktietorget
2008
- Oktober 31, 2008 - Delårsrapport 2008-01-01 till 2008-09-30
- September 30, 2008 - Amerikanskt universitet köper In Vitro Magnetic Field Generator.
- Augusti 29, 2008 - Halvårsrapport 2008-01-01 till 2008-06-30
- June 30, 2008 - In Vitro Magnetic Field Generator erhåller CE-godkännande.
- Maj 16, 2008 - Kommuniké årsstämma 2008
- April 14, 2008 - Kvartalsrapport 2008-01-01 till 2008-03-31
- Januari 21, 2008 - Bokslutskommuniké för 2007
2007
- Oktober 26, 2007 - Delårsrapport 2007-01-01 till 2007-09-30
- October 16, 2007 - EXIT i Genovis medförde kassaförstärkning på 2,9 Mkr samt vinstprognos för verkamhetsåret 2007.
- August 31, 2007 - Halvårsrapport 2007-01-01 till 2007-06-30
- July 12, 2007 - Aktieförsäljning medförde kassaförstärkning på 1,37 Mkr.
- July 10, 2007 - Byter sina samtliga A-aktier mot B-aktier i Genovis AB
- Maj 21, 2007 - Förvärvar tomt på Brunnshög i Lund för 1,38 MKr
- April 30, 2007 - Dagordning/förslag inför årsstämma 2007
- April 30, 2007 - Kvartalsrapport 2005-01-01 till 2005-03-31
- February 13, 2007 - Bokslutskommuniké för 2006
- Januari 17, 2007 - Genovisförsäljning medförde kassaförstärkning på 0,31 Mkr
2006
- December 5, 2006 - Kassaförstärkning på 0,41 MKr och uppjusterad vinstprognos för 2006
- November 30, 2006 - Delårssrapport 2006-01-01 till 2006-09-30
- August 31, 2006 - Halvårsrapport 2006-01-01 till 2006-06-30
- Maj 2, 2006 - Material inför årsstämma samt korrigering
- April 28, 2006 - Kvartalsrapport 2006-01-01 till 2006-03-31
- Mars 28, 2006 - Kassaförstärkning på 0,8 Mkr genom avyttring
- February 13, 2006 - Bokslutskommuniké för 2005
2005
- November 29, 2005 - Delårsrapport 2005-01-01 till 2005-09-30
- November 11, 2005 - Chemel förstärker kassan med 17 Mkr
- August 29, 2005 - Halvårsrapport 2005-01-01 till 2005-06-30
- May 27, 2005 - Kvartalsrapport 2005-01-01 till 2005-03-31
- April 27, 2005 - Kallelse till ordinarie bolagsstämma 2005-05-26
- April 4, 2005 - NGM erbjudande 1 utfall 2005
- February 17, 2005 - Bokslutskommuniké 2004-01-01 till 2004-12-31
2004
- November 30, 2004 - Delårsrapport 2004-01-01 -> 2004-09-30
- November 10, 2004 - Nyemission förstärker kassa med 1,4 Mkr
- September 20, 2004 - Nyemission på 4.5 MKR beslutad på (2)
- September 20, 2004 - Nyemission på 4.5 MKR beslutad (1)
- September 2, 2004 - Kallelse till extra bolagsstämma
- August 31, 2004 - Halvårsrapport 2004-01-01 -> 2004-06-30
- May 6, 2004 - Beslut tagna på ordinarie bolagstämma
2003
- November 26, 2003 - Delårsrapport 2003-01-01 -> 2003-09-30
- May 21, 2003 - Chemel´s nyemission tecknades till 80 %
- April 11, 2003 - Kvartalsrapport 2003-01-01-> 2003-03-31
- February 13, 2003 - Implementa Hebe avtal m. Labora, patent i USA
- January 21, 2003 - Företrädesemission på 9 MKR beslutad